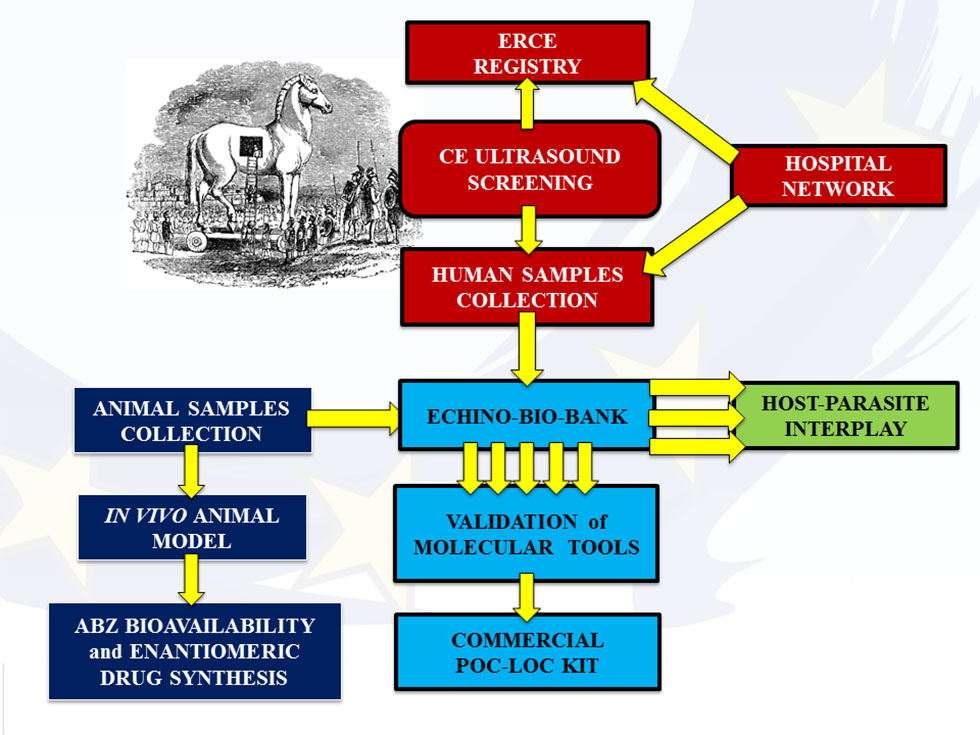
Project work packages
![]() WP1: Ultrasound screening of Eastern European population and CE registries
WP1: Ultrasound screening of Eastern European population and CE registries
![]() WP1: Ultrasound screening of Eastern European population and CE registries
WP1: Ultrasound screening of Eastern European population and CE registries
This research will systematically study the effectiveness, rate of adverse reactions, relapse rate and costs of different clinical management approaches implemented according to WHO-IWGE (Informal Working Group on Echinococcosis) guidelines. Thus ultrasound surveys for CE case detection and data collection will be organized in Romania, Bulgaria and Turkey. Patients will give their informed consent according to the national and international legislation on data privacy. Data will be recorded on a case report form which will include a questionnaire with an epidemiologic section to be answered by the participants and a clinical section to be completed by the reporting physician. Data will be collected from both patients examined in the referral hospitals as well as those identified by the active case finding through US screening. These data will be collected into European Registry of Cystic Echinococcosis (ERCE) that will provide a more detailed picture of the prevalence and actual parasitic pressure on Eastern European populations.
Study results will not only provide the policymakers with crucial information concerning the need to plan control programs, but will also disseminate internationally agreed practices on rational selection of treatment.
![]() WP2 New molecular-based tools for the detection, diagnosis and follow-up of CE
WP2 New molecular-based tools for the detection, diagnosis and follow-up of CE
![]() WP2 New molecular-based tools for the detection, diagnosis and follow-up of CE
WP2 New molecular-based tools for the detection, diagnosis and follow-up of CE
Widely accepted and routinely applied methods for the diagnosis of E. granulosus in intermediate hosts are limited in number and quality and those available have never been properly validated. The current serological methods for human CE are mainly based on the detection of IgG antibodies and partially purified native antigens against hydatid cyst fluid (HCF), either by ELISA or by immunoblot. The overall objective of WP2 is to improve and standardize serological CE detection and diagnostic/follow-up kits to improve the clinical management of CE.
This will be done by: i) establishing a representative repository of genetic Echinococcus granulosus complex isolates and serum/plasma samples collected from human patients with CE; ii) creating an Echino-Bio-bank for validating diagnostic kits; iii) validating recombinant proteins to use in diagnostic kits for immunological surveillance, diagnosis and follow-up; iiii) translating the research results into affordable and easy to use Point-of-Care/Lab-on-a-Chip (PoC/LoC) commercialized kits for its use in less favoured Eastern European countries affected by CE.
![]() WP3 Host-parasite interplay
WP3 Host-parasite interplay
![]() WP3 Host-parasite interplay
WP3 Host-parasite interplay
The host’s side: Several genetic systems are involved in the regulation of the immune response but two are widely recognized as the most important players of the immunological orchestra: i) HLA (Human Leukocyte Antigens) genes, and ii) KIR (Killer cell Immunoglobulin-like Receptors). We will study both these genetic systems mainly in their epistatic interaction.
The Parasite’s side: Echinococcal Cysts will be identified at haplotype level within E. granulosus sensu stricto and genotype/species within E. granulosus complex. To study the molecular basis of the host-parasite interaction, and to gain understanding of the Egc virulence, we will analyze the comparative proteomic from different cyst stages. Moreover, the exosomes (membrane-enclosed microvesicles, Mvs) in plasma of CE patients will be studied using the proteomic approach.
We will combine these studies on both parasite and its human host, arguing that overlapping “omic” approaches, KIR/HLA polymorphism and clinical picture of patients could provide insights on the clinical course of disease, and may be predictive of the efficacy of a drug-based treatment. The main aim of WP3 is to identify factors related to CE pathogenicity.
More in details, we will: i) Identify the molecular bases related to the pathogenicity of this disease; ii) Study the natural history of cysts and investigate the reasons behind the poor response to non surgical treatments of some cyst stages in the liver.
![]() WP4 Increasing bioavailability of ABZ and synthesis of a new enantiomeric drug
WP4 Increasing bioavailability of ABZ and synthesis of a new enantiomeric drug
![]() WP4 Increasing bioavailability of ABZ and synthesis of a new enantiomeric drug
WP4 Increasing bioavailability of ABZ and synthesis of a new enantiomeric drug
Antiparasitic drugs are playing an increasingly defined role in the management of CE. The benzimidazoles (BZs), such as albendazole (ABZ) and mebendazole (MBZ), are the only available drugs for treating uncomplicated CE cysts and are an alternative to invasive surgery in selected cases. This research will determine the content of single enantiomers of ABZSO in human/animal plasma by HPLC on polysaccharide-based chiral stationary phases. An effective HPLC system for enantio-separation on semi-preparative scale will also be developed.
The aim of WP4 is to: i) determine how HP-βCD improves the oral bioavailability of ABZ compounds; ii) synthesize a new enantiomeric drug based on ABZ.
The improved formulation, that will be tested in an animal model, may lead to the development of a new drug formulation to use in patients. Therefore the HERACLES consortium will also implement a phase 1 clinical trial protocol taking into account the pharmacokinetics, safety, and tolerability of the improved formulation.
![]() WP5 Dissemination, Training and Exploitation
WP5 Dissemination, Training and Exploitation
![]() WP5 Dissemination, Training and Exploitation
WP5 Dissemination, Training and Exploitation
Dissemination of knowledge generated by HERACLES, beyond the project partners, will cover three levels: (i) EU citizens (general public), especially the EEP living in rural areas), (ii) general practitioners, clinicians and biologists (iii) health authorities and policy makers at regional, national and EU levels.
Training activities will be the following:
- Exchange of key staff members between labs to enhance cohesion and improve implementation of project activities, and to share skills and expertise.
- Short ultrasound training courses focused on the recognition of echinococcal cysts (Focused Assessment with Sonography for CE, FASE) for general practitioners.
- Percutaneous treatment (PAIR and related techniques) training courses for specialist physicians, in the EEC where the surveys will be conducted.
- Training courses for veterinarians.
- Participation of scientists of HERACLES to national and international scientific courses, workshops and congresses.
- Educational events directed to the rural populations will be organized especially during the surveys.
Exploitation of the project results: a new molecular-based POC-LOC kits for immunological surveillance, quick diagnosis and follow-up in humans and animals will be validated within the project. The industrial partner, the Spanish SME Vircell responsible of the implementation of the POC-LOC kits is part of the consortium.
![]() WP6 Management and IPR
WP6 Management and IPR
![]() WP6 Management and IPR
WP6 Management and IPR
The main objective of WP6 is to ensure an efficient and correct project management and coordination of the whole project. This will be done by appropriate administrative and financial management which also ensures timely delivery of project reports and deliverables. The Intellectual Property Rights (IPR) on products generated by the project will be managed and protected. A Consortium Agreement, defining the Consortium members’ rights and duties and to handle IPR issues arising during the project, was signed before the project start.
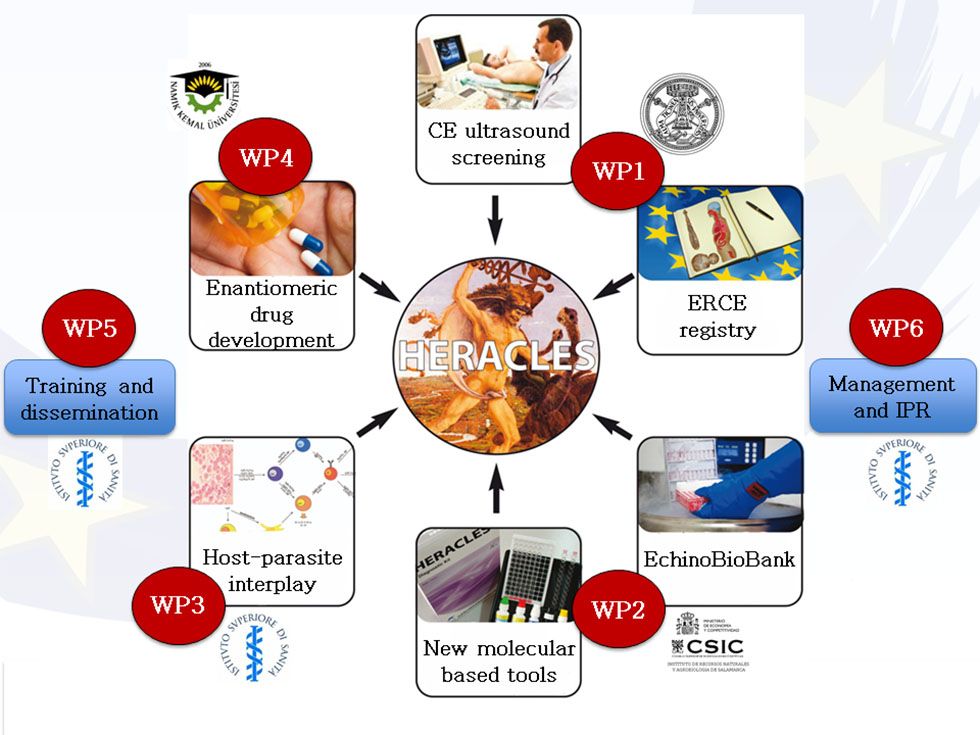
HERACLES workflow